Bottom line: maybe
I meant to post last week about the lackluster data that convalescent plasma from patients recovered from Covid-19 was effective for hospitalized patients with the virus. Here is the summary from Journal Watch dated August 19th:
Plasma authorization halted: FDA emergency authorization for convalescent plasma as a treatment for COVID-19 is now on hold, the New York Times reports. National Institutes of Health leaders, including Drs. Francis Collins, Anthony Fauci, and H. Clifford Lane, said data from the largest trial to date — encompassing over 35,000 infused patients — are insufficient to justify approval, according to the Times. The results suggested that plasma lowered mortality in hospitalized patients when given within 3 days of diagnosis, but whether treating patients that early is realistic remains to be seen. In addition, there was no placebo group. The trial has enrolled over 66,000 patients thus far.
Much to my surprise, I heard on the news last night that it was granted Emergency Use Authorization by the FDA. This was announced by President Trump. Here is the FDA’s press release:
FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising COVID–19 Treatment, Another Achievement in Administration’s Fight Against Pandemi
- For Immediate Release:
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients as part of the agency’s ongoing efforts to fight COVID-19. Based on scientific evidence available, the FDA concluded, as outlined in its decision memorandum, this product may be effective in treating COVID-19 and that the known and potential benefits of the product outweigh the known and potential risks of the product.
Today’s action follows the FDA’s extensive review of the science and data generated over the past several months stemming from efforts to facilitate emergency access to convalescent plasma for patients as clinical trials to definitively demonstrate safety and efficacy remain ongoing.
The EUA authorizes the distribution of COVID-19 convalescent plasma in the U.S. and its administration by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19 in hospitalized patients with COVID-19.
Alex Azar, Health and Human Services Secretary:
“The FDA’s emergency authorization for convalescent plasma is a milestone achievement in President Trump’s efforts to save lives from COVID-19,” said Secretary Azar. “The Trump Administration recognized the potential of convalescent plasma early on. Months ago, the FDA, BARDA, and private partners began work on making this product available across the country while continuing to evaluate data through clinical trials. Our work on convalescent plasma has delivered broader access to the product than is available in any other country and reached more than 70,000 American patients so far. We are deeply grateful to Americans who have already donated and encourage individuals who have recovered from COVID-19 to consider donating convalescent plasma.”
Stephen M. Hahn, M.D., FDA Commissioner:
“I am committed to releasing safe and potentially helpful treatments for COVID-19 as quickly as possible in order to save lives. We’re encouraged by the early promising data that we’ve seen about convalescent plasma. The data from studies conducted this year shows that plasma from patients who’ve recovered from COVID-19 has the potential to help treat those who are suffering from the effects of getting this terrible virus,” said Dr. Hahn. “At the same time, we will continue to work with researchers to continue randomized clinical trials to study the safety and effectiveness of convalescent plasma in treating patients infected with the novel coronavirus.”
Scientific Evidence on Convalescent Plasma
Based on an evaluation of the EUA criteria and the totality of the available scientific evidence, the FDA’s Center for Biologics Evaluation and Research determined that the statutory criteria for issuing an EUA criteria were met.
The FDA determined that it is reasonable to believe that COVID-19 convalescent plasma may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients. The agency also determined that the known and potential benefits of the product, when used to treat COVID-19, outweigh the known and potential risks of the product and that that there are no adequate, approved, and available alternative treatments.
The EUA is not intended to replace randomized clinical trials and facilitating the enrollment of patients into any of the ongoing randomized clinical trials is critically important for the definitive demonstration of safety and efficacy of COVID-19 convalescent plasma. The FDA continues to recommend that the designs of ongoing randomized clinical trials of COVID-19 convalescent plasma and other therapeutic agents remain unaltered, as COVID-19 convalescent plasma does not yet represent a new standard of care based on the current available evidence.
Terms of EUA
The EUA requires that fact sheets providing important information about using COVID-19 convalescent plasma in treating COVID-19 be made available to health care providers and patients, including dosing instructions and potential side effects. Possible side effects of COVID-19 convalescent plasma include allergic reactions, transfusion-associated circulatory overload, and transfusion associated lung injury, as well as the potential for transfusion-transmitted infections.
Mayo Clinic Expanded Access Program
The FDA initially facilitated access to convalescent plasma for treating COVID-19 by using pathways that included traditional clinical trials and emergency single-patient investigational new drug (IND) applications.
An Expanded Access ProgramExternal Link Disclaimer for convalescent plasma was initiated in early April to fill an urgent need to provide patient access to a medical product of possible benefit during a time that the FDA was working with researchers to facilitate the initiation of randomized clinical trials to study convalescent plasma. As the number of single patient IND requests started to number in the hundreds on a daily basis, the FDA worked collaboratively with industry, academic, and government partners to implement an expanded access protocol to provide convalescent plasma to patients in need across the country via the national expanded access treatment protocol. The program was developed with funding from the HHS’ Biomedical Advanced Research and Development Authority (BARDA), with the Mayo Clinic serving as the lead institution. To date, the program has facilitated the infusion of over 70,000 patients with convalescent plasma.
The EUA was issued to the HHS Office of the Assistant Secretary for Preparedness and Response.
The EUA remains in effect until the termination of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19. The EUA may be revised or revoked if it is determined the EUA no longer meets the statutory criteria for issuance.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Here is the study from the Mayo Clinic which has not yet been peer-reviewed.https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1.full.pdf
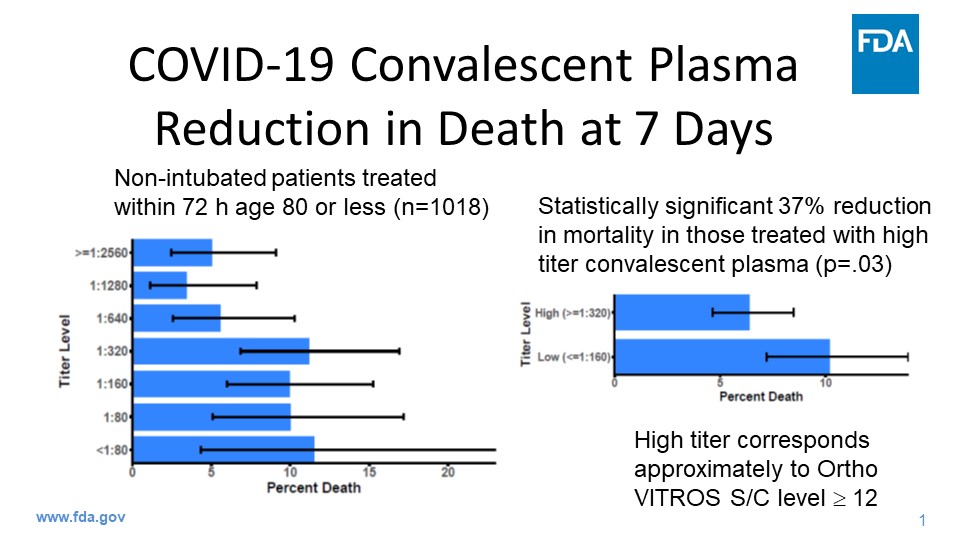
Here is a summary from Journal Watch:
Emergency authorization for convalescent plasma: Convalescent plasma has been granted emergency use authorization for treating hospitalized adults with COVID-19, the FDA announced Sunday. This follows a study from the Mayo Clinic that suggested a mortality benefit with convalescent plasma. In 35,000 patients hospitalized with COVID-19, 7-day mortality was 9% in those transfused within 3 days of their diagnosis, and 12% if transfused 4 or more days later. Use of convalescent plasma with higher antibody levels was also associated with lower mortality than plasma with lower antibody levels, with a roughly 35% reduction in overall mortality. The study did not have a traditional placebo arm and has not undergone peer review. Last week, top NIH officials cautioned against emergency approval, saying that the data were not strong enough.
So what happened? Did the FDA, which has been harshly criticized by the President, bow to political pressure? I don’t know. Certainly this treatment deserves further scrutiny and should be compared with placebo to know whether it is truly effective. These results of available studies do not show that it is a “a powerful therapy”. As with other therapies touted by political figures, it makes me uncomfortable when politics mixes with medicine.
Thank you to Jim Berg who is shown donating plasma for his photo.