There was some encouraging news regarding the development of a vaccine for Covid-19 from the New England Journal of Medicine. It is mentioned by it’s other name, SARS-CoV-2. I am including a summary from Journal Watch followed by a link to the original article. I encourage you to look at the original because it may give some insight into the complexity of the problem and the work that goes into a solution. But first there is a quotation that someone sent me. I don’t know the source, but I approve of it’s content. Think of it whenever you read the latest news on the internet.
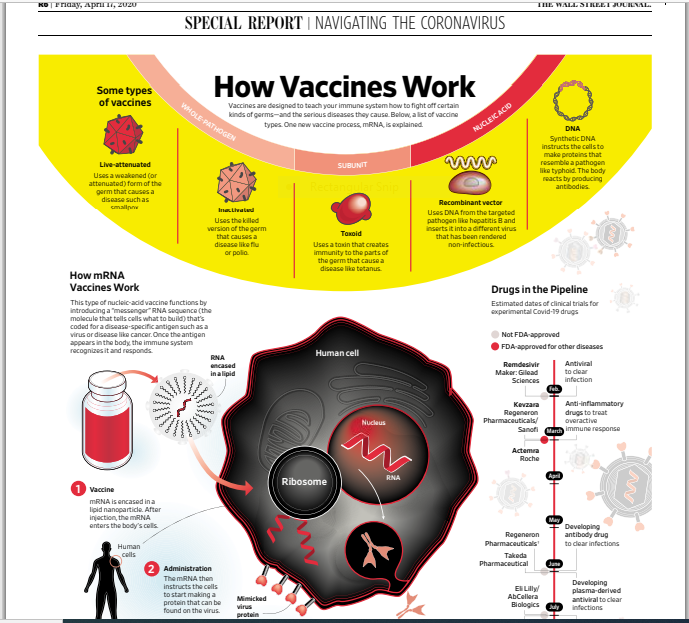
Next is a graphic that I had included on an earlier blog about how vaccines work:

July 14, 2020
Phase I SARS-CoV-2 Vaccine Trial Results Published
By Kelly Young
Edited by David G. Fairchild, MD, MPH
Moderna’s mRNA-1273 vaccine candidate against SARS-CoV-2 was largely safe and induced an immune response in humans in a phase I trial, according to results published in the New England Journal of Medicine.
Forty-five healthy adults were given two injections of the vaccine (at 25-, 100-, or 250-µg), spaced 28 days apart. All participants experienced seroconversion for binding antibodies by day 15. In the two higher doses, the median magnitude of antibody responses after the first vaccination was similar to what was observed in samples of convalescent serum. After the second vaccination, all dose groups had antibody values that were in the top quartile for convalescent serum. T-cell response was also observed.
None of the patients experienced serious adverse events, although one patient developed hives after the first dose. Systemic adverse events were more common after the second dose and occurred in all patients who received the highest dose. One patient in the 250-µg group developed a fever of 39.6° C (103° F).
The authors note that a phase II trial of this vaccine is ongoing, and a phase III trial of the 100-µg dose is expected to begin this summer.
An editorialist writes: “Accelerating the development of Covid-19 vaccine candidates beyond phase 1 depends on continued parallel tracking of activities and fulsome resources. The world has now witnessed the compression of 6 years of work into 6 months. Can the vaccine multiverse do it again, leading to a reality of a safe, efficacious Covid-19 vaccine for the most vulnerable in the next 6?”
Here’s the original:
https://www.nejm.org/doi/full/10.1056/NEJMoa2022483?query=RP
Best practices occur by incorporating the latest medical research into one’s clinical specialty and using it in the treatment of patients. What you don’t want, even in a pandemic, is your physician practicing medicine based on a you tube video of someone in West Texas who treated a bunch of people with a drug and they all got better and none of them died. That’s not how best practices evolve. Here’s my favorite you tube clip of what you don’t want from West Texas or more precisely what don’t you want.
https://www.youtube.com/watch?v=gdYXQ-IPZcc
From Hell or High Water.
Happy Bastille Day!