How will we determine whether a vaccine is safe and effective? In part by reviewing evaluations by independent organizations like The Medical Letter. Here is their analysis of two of the vaccines which are most likely to be approved in the near future. This indicates that they are effective and have a low incidence of side effects, none of which are unusual in vaccines.
ADVANCED
RELEASE
ARTICLE
The FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) will review two requests for Emergency Use Authorization (EUA) of COVID-19 vaccine candidates this month. They will review Pfizer and BioNTech’s request on December 10 and Moderna’s request on December 17. Both meetings will be webcast from the FDA’s website and streamed on Facebook, Twitter, and YouTube.1,2
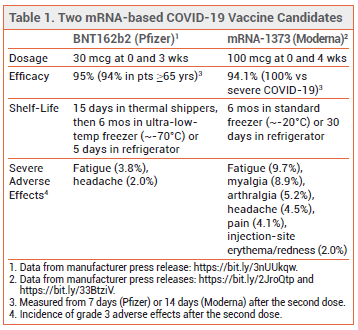
THE PFIZER/BIONTECH VACCINE — A Pfizer press release described the results of a double-blind trial in which 43,661 subjects were randomized 1:1 to receive 30 mcg of the mRNA-based COVID-19 vaccine candidate (BNT162b2) or placebo at 0 and 3 weeks. COVID-19 occurred in 8 persons who received the vaccine and in 162 who received placebo; the vaccine efficacy rate (measured from 7 days after the second dose) was 95%. Among adults ≥65 years old, the vaccine efficacy rate was 94%. Severe COVID-19 occurred in 1 subject who received the vaccine and in 9 who received placebo. The most common grade 3 adverse effects of the vaccine were fatigue (3.8%) and headache (2.0%); older adults generally reported fewer and milder adverse reactions than other vaccine recipients.3
In the US, Pfizer plans to deliver the vaccine directly to points of use in temperature-controlled thermal shippers. Upon arrival, doses can be left in the thermal shippers for up to 15 days, with dry ice periodically replenished to maintain a storage temperature of -70°C±10°C. Subsequently, the vaccine can either be stored in a standard refrigeration unit for an additional 5 days or preserved in an ultra-low-temperature freezer for up to 6 months. Doses cannot be refrozen once thawed. Pfizer and BioNTech expect to produce about 1.3 billion vaccine doses worldwide by the end of 2021.1,4
THE MODERNA VACCINE — A Moderna press release detailed top-line findings from a double-blind trial (COVE) in which >30,000 adult subjects were randomized 1:1 to receive 100 mcg of the vaccine candidate (mRNA-1273) or placebo at 0 and 4 weeks. COVID-19 occurred in 11 persons who received the vaccine and in 185 who received placebo; the vaccine efficacy rate (measured from 14 days after the second dose) was 94.1%. Thirty cases of severe COVID-19 (one fatal) occurred in the placebo group, compared to none in the vaccine group.5 Grade 3 adverse effects reported with use of the vaccine include fatigue, myalgia, arthralgia, headache, pain, and injection-site erythema/redness (see Table 1).6
The Moderna vaccine has a 6-month shelf-life at standard freezer temperatures (-20°C±5°C). Within those 6 months, it can be thawed and stored in a refrigerator for up to 30 days. After being removed from refrigeration, the vaccine must be used within 12 hours.7
In the US, distribution of Moderna’s vaccine will be supported by the federal Operation Warp Speed project. Vaccine doses and supplies will be staged at McKesson distribution centers and then delivered directly to points of use.8 Moderna expects to have 20 million doses available in the US by the end of 2020 and to produce 0.5-1 billion doses worldwide by the end of 2021.5
VACCINE PRIORITY — Vaccinations will probably begin within days after the issuance of an EUA. The ACIP has recommended that healthcare personnel and long-term care facility residents are the first to be immunized.9
- FDA News Release. Coronavirus (COVID-19) update: FDA announces advisory committee meeting to discuss COVID-19 vaccine candidate. November 20, 2020. Available at: https://bit.ly/3781bpx. Accessed December 3, 2020.
- FDA News Release. Coronavirus (COVID-19) update: FDA announces advisory committee meeting to discuss second COVID-19 vaccine candidate. November 30, 2020. Available at: https://bit.ly/39v4s5i. Accessed December 2, 2020.
- Pfizer. Pfizer and BioNTech conclude phase 3 study of COVID-19 vaccine candidate, meeting all primary efficacy endpoints. November 18, 2020. Available at: https://bit.ly/3nUUkqw. Accessed December 2, 2020.
- Pfizer. Pfizer-BioNTech COVID-19 vaccine U.S. distribution fact sheet. November 20, 2020. Available at: https://bit.ly/37bL6zw. Accessed December 3, 2020.
- Moderna. Moderna announces primary efficacy analysis in phase 3 COVE study for its COVID-19 vaccine candidate and filing today with U.S. FDA for Emergency Use Authorization. November 30, 2020. Available at: https://bit.ly/2JroQtp. Accessed December 3, 2020.
- Moderna. Moderna’s COVID-19 vaccine candidate meets its primary efficacy endpoint in the first interim analysis of the phase 3 COVE Study. November 16, 2020. Available at: https://bit.ly/33BtziV. Accessed December 3, 2020.
- Moderna. Moderna announces longer shelf life for its COVID-19 vaccine candidate at refrigerated temperatures. November 16, 2020. Available at: https://bit.ly/2Jp4ila. Accessed December 3, 2020.
- HHS. Fact sheet: explaining Operation Warp Speed. Available at: https://bit.ly/3fZso1G. Accessed December 3, 2020.
- CDC Advisory Committee on Immunization Practices (ACIP). ACIP presentation slides: December 2020 meeting. December 1, 2020. Available at: https://bit.ly/37wZx1f. Accessed December 3, 2020.
![]()