The illustration looks suspiciously like Covid 19, but is RSV, respiratory syncytial virus, another nasty respiratory pathogen. There are two new vaccines that are available to prevent it, but it has been tough to tell from the published data which one is more effective and has fewer side effects. I was hoping that The Medical Letter, an independent publication that is uninfluenced by the pharmaceutical industry, would weigh in on this and they have. The bottom line is that they are both similar in efficacy, but Abrysvo has fewer side effects when compared to placebo than Arexvy.
They are pricey and only effective for one or two years. They are most appropriate for elderly patients with underlying medical problems. Here are the details:
- Description: Both are recombinant vaccines containing the prefusion form of the RSV fusion (F) glycoprotein.
- Indication: Both are FDA-approved for prevention of lower respiratory tract disease (LRTD) caused by RSV in adults ≥60 years old. Abrysvo is also approved for use in pregnant women at 32-36 weeks’ gestation to prevent LRTD caused by RSV in their infants from birth through 6 months of age.
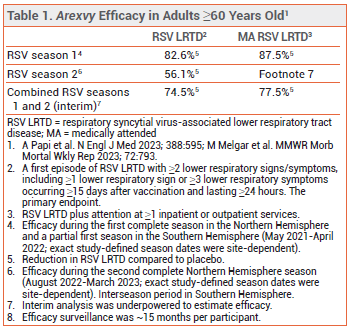
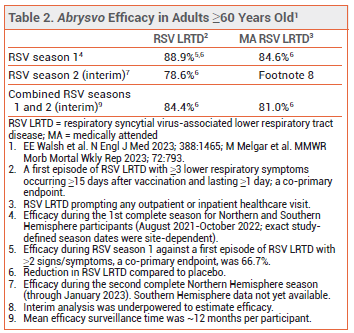
- Efficacy: Both reduced the incidence of RSV-associated LRTD in adults ≥60 years old during 2 RSV seasons in the Northern Hemisphere. Use of Abrysvo in pregnant women reduced the incidence of medically attended severe RSV-associated LRTD in their infants during one RSV season.
- Adverse Effects: Most common are injection-site reactions, fatigue, headache, and myalgia. Guillain-Barré syndrome (GBS) and atrial fibrillation were reported in older adults.
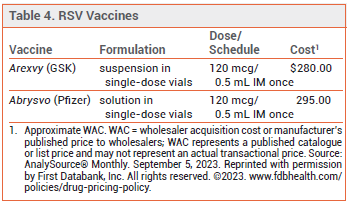
- Dosage: A single 120 mcg/0.5 mL IM dose.
- Cost: The cost for one dose is $280 (Arexvy) or $295 (Abrysvo).
- Conclusion: The CDC Advisory Committee on Immunization Practices (ACIP) recommends use of either vaccine in adults ≥60 years old at highest risk for severe RSV disease. Giving Abrysvo to pregnant women can reduce the risk of severe RSV disease in their infants; giving a single dose of the long-acting monoclonal antibody nirsevimab (Beyfortus) to infants is another effective option.
Outline
Tables |
Two recombinant vaccines, Arexvy (GSK) and Abrysvo (Pfizer), have been approved by the FDA for prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in adults ≥60 years old. They are the first RSV vaccines to be approved in the US. Abrysvo is also approved for use in pregnant women at 32-36 weeks’ gestation to prevent LRTD caused by RSV in their infants from birth through 6 months of age.
RSV DISEASE ― RSV typically causes a mild upper respiratory tract infection, but infants <6 months old and older adults, particularly those with underlying health conditions, have an increased risk of hospitalization due to RSV-associated LRTD. RSV infections cause 58,000-80,000 hospitalizations among children <5 years old and 60,000-160,000 among adults ≥65 years old each year in the US. Both major subtypes of RSV (RSV-A and RSV-B) circulate each season, but the dominant subtype varies. RSV epidemics in the Northern Hemisphere typically occur between October and April, peaking in December or January. The COVID-19 pandemic disrupted RSV seasonality, but prepandemic seasonal patterns are returning.1

Prevention – No drug is approved for prevention of RSV infection in adults. The monoclonal antibody palivizumab (Synagis) has been available for many years for prevention of RSV disease in high-risk children <24 months old; it is given monthly during the RSV season. Nirsevimab (Beyfortus), a long-acting monoclonal antibody given in a single dose, is now FDA-approved to provide protection against RSV disease for all infants during their first RSV season.2
THE VACCINES ― The antigen in both recombinant vaccines is the prefusion form of the RSV fusion (F) glycoprotein, which mediates viral fusion and host-cell entry and elicits neutralizing antibodies. Arexvy (RSVPreF3) contains the prefusion RSV F antigen of an RSV-A strain and an adjuvant (AS01E). Abrysvo (RSV preF) is a bivalent vaccine that contains equal amounts of stabilized prefusion F antigens from RSV-A and RSV-B.
CLINICAL STUDIES ― Adults ≥60 years old – In ongoing trials in 24,966 participants (Arexvy) and 34,284 participants (Abrysvo), adults ≥60 years old were randomized to receive a single dose of vaccine or placebo shortly before or (Abrysvo only) during the RSV season and are being followed for 2 (Abrysvo) or 3 (Arexvy) RSV seasons. One dose of either vaccine reduced the incidence of a first episode of confirmed RSV-associated LRTD or medically attended RSV occurring ≥15 days after vaccination, compared to placebo, for up to 2 RSV seasons (see Tables 1 and 2).3-5
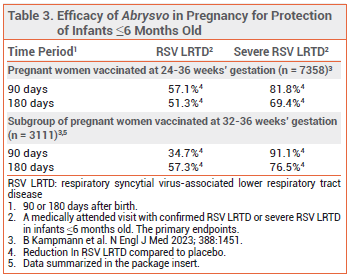
Pregnant women – In an ongoing, randomized, double-blind trial (MATISSE), 7358 pregnant women received a single dose of Abrysvo or placebo at 24-36 weeks’ gestation. Vaccination with Abrysvo significantly reduced the incidence of medically attended RSV-associated LRTD and severe LRTD in their infants ≤6 months old, compared to placebo (see Table 3).6
Administration with Other Vaccines – In studies in which Arexvy or Abrysvo was given with a quadrivalent seasonal influenza vaccine, RSV and influenza antibody titers were generally somewhat lower with coadministration than with sequential administration (~1 month apart).5 When Abrysvo was administered concomitantly with a tetanus, diphtheria, and acellular pertussis (Tdap) vaccine in nonpregnant persons 18-49 years old, geometric mean antibody concentrations to the acellular pertussis antigens were reduced compared to administration of Tdap alone. The clinical significance of these reductions is unknown.
ADVERSE EFFECTS ― Adults ≥60 years old – Local and systemic adverse effects that occurred within 4 days of vaccination and more often with Arexvy than placebo in the pivotal clinical trial included injection-site pain (60.9% vs 9.3%), erythema (7.5% vs 0.8%) and swelling (5.5% vs 0.6%), fatigue (33.6% vs 16.1%), myalgia (28.9% vs 8.2%), headache (27.2% vs 12.6%), arthralgia (18.1% vs 6.4%), and fever (2.0% vs 0.3%). Guillain-Barré syndrome (GBS) occurred in one patient 9 days after vaccination with Arexvy. Within 30 days after vaccination, atrial fibrillation occurred more often with Arexvy than with placebo (10 vs 4 cases).
Local reactions reported within 7 days of vaccination and more often with Abrysvo than with placebo included injection-site pain (10.5% vs 6.0%), erythema (2.7% vs 0.7%) and swelling (2.4% vs 0.5%), fatigue (15.5% vs 14.4%), headache (12.8% vs 11.7%), and myalgia (10.1% vs 8.4%). Serious adverse events considered possibly related to the vaccine included one hypersensitivity reaction 8 hours after vaccination, and one case each of GBS and Miller Fisher syndrome (a variant of GBS) 10-14 days after vaccination.5 Within 30 days after vaccination, atrial fibrillation occurred more frequently with Abrysvo than with placebo (10 vs 4 cases).
Pregnant women – Adverse effects that were reported more often in Abrysvo recipients than placebo recipients in the MATISSE trial included injection-site pain (40.6% vs 10.1%), erythema (7.2% vs 0.2%) and swelling (6.2% vs 0.2%), headache (31.0% vs 27.6%), and myalgia (26.5% vs 17.1%). Pre-eclampsia occurred in 1.8% of pregnant women who received the vaccine compared to 1.4% of those who received placebo. The labeling recommends administering the vaccine at 32-36 weeks’ gestation because preterm births were more common with Abrysvo than with placebo (5.7% vs 4.7%); a causal relationship has not been established.
Higher rates of low birth weight and jaundice were observed in the infants of mothers who received Abrysvo (5.1% and 7.2% vs 4.4% and 6.7% with placebo).
ACIP RECOMMENDATIONS ― The CDC Advisory Committee on Immunization Practices (ACIP) recommends that until more safety data become available, RSV vaccination with a single dose of Arexvy or Abrysvo should be targeted to adults ≥60 years old at highest risk for severe RSV disease, which includes those with pulmonary or cardiovascular diseases, moderate or severe immune compromise, diabetes, neurologic or neuromuscular conditions, renal or hepatic impairment, or hematologic disorders. Frailty, advanced age, and residence in a long-term care facility are also associated with an increased risk of severe RSV disease.
For optimal protection, vaccination should occur before the onset of the RSV season. The vaccines are already available for the 2023-24 season; vaccination should be offered immediately to eligible adults and continue to be offered to those who remain unvaccinated because some off-season RSV circulation is possible.
Coadministration of RSV vaccines and other adult vaccines during the same visit is acceptable, but data are limited. Administering RSV vaccines with other vaccines might increase local or systemic reactogenicity.5
The ACIP has not yet issued recommendations for use of Abrysvo in pregnant women. The ACIP has recommended a single IM dose of nirsevimab for all infants born during or entering their first RSV season.2
DOSAGE AND ADMINISTRATION ― The antigenic component in Arexvy is supplied as a lyophilized powder in a single-dose vial that must be reconstituted with the adjuvant, which is supplied as a suspension in a separate vial; before reconstitution, the vials should be stored in the refrigerator in the original package. After reconstitution, the dose (120 mcg RSVPreF3/0.5 mL) can be stored in the refrigerator or at room temperature for up to 4 hours.
Abrysvo is supplied in kits that include a vial of antigen (lyophilized powder), a pre-filled syringe containing the diluent (sterile water), and a vial adapter. The kit should be stored in the refrigerator in the original carton before reconstitution. After reconstitution, the dose (60 mcg RSV preF A and 60 mcg RSV preF B/0.5 mL) can be stored at room temperature for up to 4 hours.
CONCLUSION ― Arexvy and Abrysvo are the first respiratory syncytial virus (RSV) vaccines to be approved by the FDA. A single dose of either vaccine appears to be effective in preventing RSV-associated lower respiratory tract disease for one, and possibly two, RSV seasons in adults ≥60 years old. Atrial fibrillation and inflammatory neurologic events such as Guillain-Barré syndrome have been reported following vaccination. Until more safety data become available, the CDC Advisory Committee on Immunization Practices (ACIP) recommends targeting vaccination to older adults at highest risk for severe RSV disease.
Vaccination of pregnant women with Abrysvo can prevent severe RSV disease in their infants ≤6 months old. The ACIP has not yet issued recommendations for use of the vaccine in this population.
- S Hamid et al. Seasonality of respiratory syncytial virus – United States, 2017-2023. MMWR Morb Mortal Wkly Rep 2023; 72:355. doi:10.15585/mmwr.mm7214a1
- Nirsevimab (Beyfortus) for prevention of severe RSV disease in young children. Med Lett Drugs Ther 2023; 65:145.
- EE Walsh et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388:1465. doi:10.1056/nejmoa2213836
- A Papi et al. Respiratory syncytial virus prefusin F protein vaccine in older adults. N Engl J Med 2023; 388:595. doi:10.1056/nejmoa2209604
- M Melgar et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices – United States, 2023. MMWR Morb Mortal Wkly Rep 2023; 72:793. doi:10.15585/mmwr.mm7229a4
- B Kampmann et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med 2023; 388:1451. doi:10.1056/nejmoa2216480
Important Copyright and Disclaimer Message: The Medical Letter® publications are protected by US and international copyright laws. Forwarding, copying, or any distribution of this material without permission to a nonsubscriber is prohibited. Sharing a password with a nonsubscriber or otherwise making the contents of this site available to third parties is prohibited. By accessing and reading the attached content I agree to comply with US and international copyright law and these terms and conditions of The Medical Letter, Inc.
The Medical Letter, Inc. does not warrant that all the material in this publication is accurate and complete in every respect. The Medical Letter, Inc. and its editors shall not be held responsible for any damage resulting from any error, inaccuracy, or omission.
[/vc_column_text][/vc_column][/vc_row]