Here are two summaries of the recent recommendations to the FDA by advisory committees regarding Moderna and J=J Covid 19 vaccines. Don’t run to the pharmacy asking for it as it still requires the CDC’s advisory committee, the ACIP to give their opinion and then the FDA to approve. There is still no recommendation on mixing vaccines, yet
FDA panel recommends Moderna coronavirus booster for people 65 and older, adults at high risk
The Washington Post (10/14, A1, Johnson, Abutaleb) reports the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) “on Thursday unanimously recommended a booster dose of the Moderna coronavirus vaccine for people 65 and older and for adults who are at high risk of severe illness because of underlying conditions or exposure on the job,” a recommendation mirroring eligibility criteria for the Pfizer coronavirus booster. FDA officials now will consider the recommendation, and a CDC advisory committee is scheduled to meet on Wednesday on the data. About 70 million U.S. residents “have been fully vaccinated with the Moderna vaccine, and millions of them would be eligible for a follow-up dose six months after vaccination if the [FDA] authorizes the extra shot, which would be half the dose initially given.”
CNN (10/14, Gumbrecht) says all 19 members of the VRBPAC “supported authorizing a 50-microgram booster dose – half the size of the 100-microgram doses used in the primary series of the two-dose vaccine – at least six months after the second dose.”
The AP (10/14, Neergaard, Perrone) reports, “There’s no evidence that it’s time to open booster doses of either the Moderna or Pfizer vaccine to everybody, the panel stressed.”
FDA panel backs booster dose for all J&J COVID-19 vaccine recipientsThe New York Times (10/15, A1, LaFraniere, Weiland, Zimmer) reported that a key FDA “advisory committee voted unanimously Friday to recommend Johnson & Johnson booster shots, most likely clearing the way for all 15 million people who got the company’s one-dose coronavirus vaccine to receive a second shot.” If the FDA and the CDC “accept the recommendation, as expected, boosters could be offered by late next week.” However, “many committee members made clear that they believed Johnson & Johnson recipients might benefit from the option of a booster of the Pfizer-BioNTech or Moderna vaccine, something a top FDA official said the agency was considering.” The AP (10/15, Perrone, Neergaard) reported the FDA advisers “cited growing evidence that J&J recipients are more vulnerable to infection than people who got vaccines from competitors Pfizer or Moderna – and that most got their single dose many months ago.” The AP added, “Although Friday’s meeting is part of an ongoing evaluation of vaccine boosters, many of the experts said it makes more sense to think of J&J’s vaccine as a two-dose vaccine.” Previous studies have shown robust antibody response by combining vaccines, but this doesn’t prove their effectiveness. This study from Sweden indicates that they do work, but it the average follow up after the second dose was only 2.5 months. I don’t think it has been peer reviewed yet. The J+J vaccine is concerned very similar to the Oxford AstraZeneca vaccine. OCTOBER 18, 2021 High effectiveness of mix-and-match COVID-19 vaccines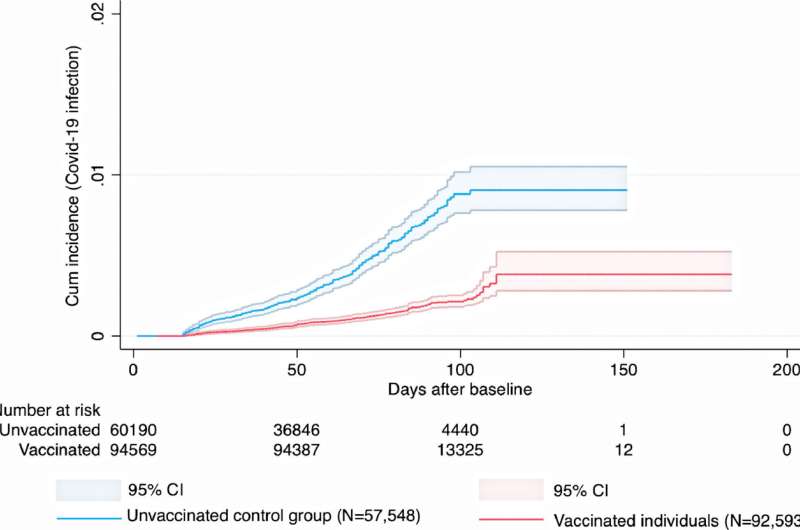 People who had received a first dose of the Oxford-AstraZeneca COVID-19 vaccine and received an mRNA vaccine for their second dose had a lower risk of infection compared to people who had received both doses of the Oxford-AstraZeneca vaccine. This is shown in a nationwide study performed by researchers at Umeå University, Sweden. “Having received any of the approved vaccines is better compared to no vaccine, and two doses are better than one,” says Peter Nordström, professor of geriatric medicine at Umeå University. “However, our study shows a greater risk reduction for people who received an mRNA vaccine after having received a first dose of a vector-based, as compared to people having received the vector-based vaccine for both doses.” Since the use of Oxford-AstraZeneca’s vector-based vaccine against COVID-19 was halted for people younger than 65 years of age, all individuals who had already received their first dose of this vaccine were recommended an mRNA vaccine as their second dose. During a 2.5-month average follow-up period after the second dose, the study showed a 67% lower risk of infection for the combination of Oxford-AstraZeneca + Pfizer-BioNTech, and a 79% lower risk for Oxford/AstraZeneca + Moderna, both compared to unvaccinated individuals. For people having received two doses of the Oxford-AstraZeneca vaccine, the risk reduction was 50%. These risk estimates were observed after accounting for differences regarding date of vaccination, age of the participants, socioeconomic status, and other risk factors for COVID-19. Importantly, the estimates of effectiveness apply to infection with the Delta variant, which was dominating the confirmed cases during the follow-up period. There was a very low incidence of adverse thromboembolic events for all vaccine schedules. The number of COVID-19 cases severe enough to result in inpatient hospitalization was too low for the researchers to be able to calculate the effectiveness against this outcome. Previous research has demonstrated that mix-and-match vaccine schedules generate a robust immune response. However, it has been unclear to which extent these schedules may reduce the risk of clinical infection. This is the knowledge gap that the new study conducted by the Umeå researchers aimed to fill. The study is based on nationwide registry data from the Public Health Agency of Sweden, the National Board of Health and Welfare, and Statistics Sweden. In the main analysis, about 700,000 individuals were included. “The results of the study may have implications for vaccination strategies in different countries,” says Marcel Ballin, doctoral student in geriatric medicine at Umeå University and co-author of the study. “The World Health Organization has stated that despite the promising results from previous studies regarding immune response from mix-and-match vaccination, there is a need for larger studies to investigate their safety and effectiveness against clinical outcomes. Here we now have one such study.” |




