Here is everything that you ever wanted to know about sunscreens. Their effectiveness, cost, pros and cons and their environmental consequences. This is a reprint of last summer’s Medical Letter on this topic. TIP: USE MORE THAN YOU THINK YOU NEED LONGER BEFORE YOU NEED IT.
The Medical Letter on Drugs and Therapeutics
ISSUE
1629
July 26, 2021
Sunscreens
Sunscreen use reduces the risk of sunburn and photoaging; regular use has been associated with a reduced risk of some skin cancers.
Adequate application (2 mg/cm2) of a broad-spectrum sunscreen with an SPF ≥15 is generally recommended.
Sunscreen should be applied 15-30 minutes before sun exposure and reapplied at least every 2 hours and after swimming or sweating.
Organic (chemical) sunscreens are absorbed systemically; whether long-term use could result in adverse health effects is unclear.
Using inorganic (physical) sunscreens such as zinc oxide and titanium dioxide is unlikely to result in systemic absorption or toxicity.
The organic sunscreens oxybenzone and octinoxate have been removed from many sunscreen products because they may be harmful to the environment.
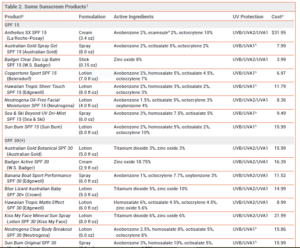
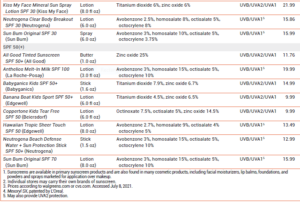
Excessive exposure to ultraviolet (UV) radiation is associated with sunburn, photoaging, and skin cancer.1,2 Sunscreens are widely used to reduce these risks, but questions remain about their effectiveness and safety. The FDA has issued a proposed rule that would require manufacturers to perform additional safety studies for some sunscreen active ingredients and would mandate better UVA protection in sunscreen products.3 Some sunscreen products containing FDA-approved active ingredients are listed in Table 2.
UVA and UVB — UV radiation capable of injuring the skin is classified based on wavelength as UVB (290-320 nm), UVA2 (320-340 nm), and UVA1 (340-400 nm). UVA, which makes up 95% of terrestrial UV radiation, penetrates the dermis and causes long-term damage. UVB, which is mostly absorbed in the epidermis, is largely responsible for the erythema of sunburn. Both UVA and UVB radiation can cause photoaging and skin cancer.4 UVB is strongest at midday; in temperate climates, it is present primarily in late spring, summer, and early autumn. UVA is constant throughout the day and the year and, unlike UVB, is not filtered by clear glass.5
SPF — The Sun Protection Factor (SPF) is the ratio of the amount of UV radiation required to produce a minimally detectable sunburn on sunscreen-protected skin to the amount required on unprotected skin. The amount of erythema-producing UV radiation (primarily UVB) that penetrates through a sunscreen product to reach the skin is affected by factors such as exposure time, intensity of solar energy, and the amount of sunscreen product applied. When properly applied, a sunscreen with an SPF of 15, 30, 50, or 100 allows 1/15, 1/30, 1/50, or 1/100, respectively, of erythemogenic UV photons to reach the skin. The percentage of erythema-producing UV radiation absorbed by sunscreens is 93% with SPF 15, 97% with SPF 30, 98% with SPF 50, and 99% with SPF 100.
There is no specific rating system in the US for how much UVA protection is provided by a sunscreen. The FDA allows sunscreens to be labeled “broad-spectrum” if they provide UVA and UVB protection and the UVA protection is proportional to the UVB protection. Manufacturers of broad-spectrum sunscreens with an SPF ≥15 can claim that their products reduce the risk of skin cancer and photoaging if used as directed with other sun protection measures. The FDA and the US Preventive Services Task Force (USPSTF) both recommend use of a broad-spectrum sunscreen with an SPF ≥15.6,7 The American Academy of Dermatology recommends use of a broad-spectrum, water-resistant sunscreen with an SPF ≥30.8
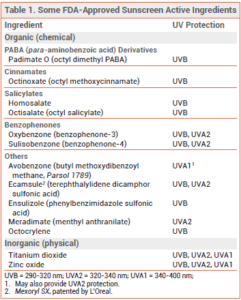
ACTIVE INGREDIENTS — Organic – Several organic (chemical) sunscreens that absorb different wavelengths of UV radiation are approved by the FDA (see Table 1). Avobenzone is an effective UVA1 absorber and it also absorbs some UVA2, but its efficacy decreases by about 60% after 60 minutes of exposure to sunlight due to photolability. Combining avobenzone with photostable UV filters improves its photostability. Oxybenzone absorbs both UVB and UVA2. Octinoxate is a potent UVB absorber. Octisalate and homosalate are weak UVB absorbers; they are generally used with other agents for additional UVB protection. Octocrylene absorbs UVB and is photostable; when combined with other sunscreens, it can improve the photostability of the entire product. Ecamsule is photostable and absorbs both UVB and UVA2.9
PABA (para-aminobenzoic acid) and trolamine salicylate are not generally recognized as safe and effective (GRASE) by the FDA and are no longer available in sunscreen products sold in the US.3
Inorganic – The two FDA-approved inorganic (mineral; physical) sunscreens, zinc oxide and titanium dioxide, block UVB, UVA2, and UVA1 penetration. Formulated as nanoparticles, zinc oxide offers better UVA protection than titanium dioxide. Used together, they provide broad UV protection.10 Nanoparticle formulations have a more cosmetically acceptable appearance and are now widely used; they are less visible on the skin, but they may also be less effective.11
Herbal Substances – Some plant-derived extracts with antioxidant effects have demonstrated photoprotective activity in vitro and in animals. Green tea extract, carotenoids such as beta-carotene, lycopene and lutein, and Polypodium leucotomos extract (PLE; derived from a South American species of fern) have been shown to reduce sunburn and improve signs of photodamage in humans, but data are limited and none of these substances are FDA-approved for sun protection.12
FORMULATIONS — Sunscreen dosage forms that the FDA has proposed to classify as GRASE include oils, lotions, creams, gels, butters, pastes, ointments, and sticks. Wipes, towelettes, body washes, and shampoos are excluded for lack of data. Sprays and powders require additional testing. Sunscreen sprays are flammable, and burns requiring hospitalization have been reported. Based on animal models, the small particles in sprays and powders could pose inhalation risks.
EFFECTIVENESS — In the amounts customarily applied to skin, no sunscreen product provides the labeled degree of protection. The FDA requires that the SPF be determined after applying 2 mg/cm2 of the product. At 2 mg/cm2, a 4-ounce container provides only 2-4 whole body applications for an adult. Studies have shown that consumers usually apply 0.5-1.0 mg/cm2 or less. Applying 0.5-mg/cm2 doses of sunscreens labeled SPF 30-100 has been shown to provide an actual SPF that is about 25% of the labeled SPF.13 Nevertheless, studies have found that long-term daily sunscreen use combined with other sun-protective measures reduces the risk of some nonmelanoma skin cancers and can reduce other adverse effects of exposure to UV radiation such as photoaging.14,15 Using a sunscreen with a high SPF may prevent DNA damage in the skin even when the sunscreen application is less than optimal.16
Prevention of Melanoma – Fair skin, use of tanning beds, and a history of sunburn are associated with increased melanoma risk.7 In an Australian population-based, case-control study that included 603 adults 18-39 years old with a first primary cutaneous melanoma diagnosis and 1088 controls 18-44 years old, regular sunscreen use in childhood and early adulthood was associated with a reduced risk of cutaneous melanoma.17 In a prospective trial, 1621 Australians 25-75 years old were randomized to use an SPF 16 sunscreen either daily or in a discretionary manner (generally 0-2 times weekly) for 4 years. Compared with discretionary users, daily sunscreen users had 50% fewer new primary melanomas (11 vs 22) and 73% fewer invasive melanomas (3 vs 11) 14 years after randomization.18
SAFETY — Organic – All organic sunscreens, especially oxybenzone, can cause contact allergic and photoallergic reactions, but severe reactions are uncommon.19 Estrogenic and anti-androgenic activities have been reported in vitro and in some animal studies.20 These agents penetrate the epidermis and are absorbed systemically; detectable levels have been reported in human plasma, urine, breast milk, amniotic fluid, and fetal and cord blood.21
Two randomized, open-label trials were conducted by the FDA to evaluate the systemic absorption of some common sunscreen active ingredients in adults.22,23 In the first trial, a sunscreen product was applied under maximal use conditions (2 mg/cm2 every 2 hours [4 times per day] to 75% of body surface area) for 4 days; the 4 active ingredients studied (3% avobenzone, 4-6% oxybenzone, 2.35-10% octocrylene, 2% ecamsule) achieved mean maximum plasma concentrations above the FDA threshold for safety testing (>0.5 ng/mL) and remained in plasma for at least 3 days after the last application.
In the second trial, the sunscreen product was applied once on day 1, followed by maximal application on days 2-4. Mean maximum plasma concentrations after a single application were >0.5 ng/mL with all 6 of the active ingredients studied (3% avobenzone, 4-6% oxybenzone, 6-10% octocrylene, 10-15% homosalate, 5% octisalate, 7.5% octinoxate); the highest levels occurred with oxybenzone (85-94 ng/mL). All of the active ingredients had long terminal half-lives (mean range 27.3-157.4 hours); concentrations of homosalate and oxybenzone were >0.5 ng/mL in >50% of participants at 21 days.
Whether such exposure could affect hormone levels or result in other adverse effects in humans is unclear.21,24 The FDA has stated that additional studies are needed to determine the clinical significance of these findings. A recent study in 441 healthy women found an association between oxybenzone exposure and urinary markers of kidney dysfunction.25
Inorganic – Studies have found that titanium dioxide and zinc oxide nanoparticles do not penetrate or minimally penetrate the stratum corneum and underlying layers of skin, suggesting that systemic absorption and toxicity are unlikely.26,27 The FDA has stated that available evidence supports a GRASE determination for zinc oxide and titanium dioxide.3
An online pharmacy (Valisure) recently tested 294 samples of commercially available sunscreens and after-sun products and found benzene, a known carcinogen, in 27% (mainly sprays); the clinical significance of this finding is unknown.28
Environmental Safety – Hawaii, Key West, the US Virgin Islands, and some island nations have passed ordinances and/or legislation banning the sale of sunscreens that contain oxybenzone and/or octinoxate because they can cause coral reef bleaching.29 The US Virgin Islands has also banned octocrylene. The FDA recently announced that it intends to evaluate the potential environmental effects associated with use of oxybenzone and octinoxate in sunscreen products.30 Detectable concentrations of sunscreen active ingredients have been observed in some fish species and adverse reproductive effects have been reported.31 Nanoparticles of zinc oxide and titanium dioxide may also have detrimental effects on the environment, including coral bleaching.32
INFANTS AND CHILDREN — Sunscreen use is generally recommended for children >6 months old during any sun exposure that might burn unprotected skin. Inorganic sunscreens are less likely than organic sunscreens to cause irritation and sensitization.33
PREGNANCY — Data on sunscreen use in pregnancy are limited. The results of human and animal studies suggest that the endocrine-disrupting effects of oxybenzone may result in reproductive toxicity; an association between maternal oxybenzone exposure and Hirschsprung’s disease in their offspring has been observed.34,35 Inorganic sunscreens are generally preferred for pregnant women.
VITAMIN D AND SUNSCREENS — The amount of sun exposure required for vitamin D synthesis is much lower than the amount that produces sunburn. Most people require only 2-8 minutes of unprotected exposure to summer sun to maximize synthesis of vitamin D3. Whether sunscreen use could lead to vitamin D3 deficiency is unclear. Two reviews have evaluated the evidence on the association between sunscreen use and vitamin D3 status. Although sunscreen use decreased vitamin D3 production in some experimental studies, most randomized, controlled field trials found no change in vitamin D3 levels with daily application of a sunscreen with an SPF of ~16.36,37 A controlled study in subjects on a 1-week sun holiday found that optimal SPF 15 sunscreen use prevented erythema and increased vitamin D production; synthesis of vitamin D was greater with use of a sunscreen product with a high UVA protection factor compared to one with a low UVA protection factor because it allowed more UVB transmission.38 No trials have evaluated the effects of high-SPF sunscreens on vitamin D3 synthesis.
APPLICATION — For maximum efficacy, sunscreen should be applied about 15-30 minutes before sun exposure and reapplied at least every 2 hours and after swimming or sweating. Water-resistant sunscreens remain effective for 40 or 80 minutes while swimming or sweating; no sunscreens are waterproof. For maximum effect, approximately one teaspoon of sunscreen should be applied to the face and neck and one to each arm; two teaspoons should be applied to the torso and two to each leg.39
With Insect Repellent – When using both a sunscreen and an insect repellent, the sunscreen should be applied first. Applying the insect repellent N,N-diethyl-m-tolumide (DEET) after sunscreen has been shown to reduce the SPF of the sunscreen, but applying sunscreen second may increase absorption of DEET. Use of products that combine a sunscreen with an insect repellent is not recommended because the sunscreen may need to be reapplied more often and in greater amounts than the repellent.
OTHER SUN PROTECTION MEASURES — In addition to sunscreen use, The American Academy of Dermatology recommends seeking shade during hours of peak sunlight (10am-2pm), and wearing sun-protective clothing, including long-sleeve shirts, pants, wide-brimmed hats, and sunglasses. Factors that affect the level of UV protection from clothing include fabric color, fabric type, and tightness of the weave. The ultraviolet protection factor (UPF) is a measure of how effective a fabric is at blocking UV radiation; UV protection is considered good with a UPF rating of 15-24, very good with a rating of 25-39, and excellent with a rating of 40-50. Washing clothes once with RIT Sun Guard, a commercially available laundry product containing a broad-spectrum UV absorber (Tinosorb FD), can confer a UPF of 30 that lasts through 20 additional washings.
HAVE A HAPPY AND SAFE MEMORIAL DAY!
REFERENCES
U Panich et al. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int 2016; 2016:7370642.
M Arnold et al. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Cancer 2018; 143:1305.
FDA, HHS. Sunscreen drug products for over-the-counter human use. A proposed rule by the Food and Drug Administration on February 26, 2019. 84(FR):6204. Available at: https://bit.ly/3jnC7CZ. Accessed July 8, 2021.
RE Neale et al. Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2020. Photochem Photobiol Sci 2021; 20:1.
F Wang et al. Dermal damage promoted by repeated low-level UVA1 exposure despite tanning response in human skin. JAMA Dermatol 2014; 150:401.
FDA. Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the U.S. June 23, 2011. Available at: https://bit.ly/3AcaeDJ. Accessed July 8, 2021.
US Preventive Services Task Force. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 319:1134.
AAD. The American Academy of Dermatology statement on the safety of sunscreen. May 22, 2019. Available at: https://bit.ly/3qzNmJT. Accessed July 8, 2021.
A new sunscreen agent. Med Lett Drugs Ther 2007; 49:41.
SL Schneider and HW Lim. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol Photoimmunol Photomed 2019; 35:442.
JB Mancuso et al. Sunscreens: an update. Am J Clin Dermatol 2017; 18:643.
L Rabinovich and V Kazlouskaya. Herbal sun protection agents: human studies. Clin Dermatol 2018; 36:369.
H Ou-Yang et al. High-SPF sunscreens (SPF ≥70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol 2012; 67:1220.
M Sander et al. The efficacy and safety of sunscreen use for the prevention of skin cancer. CMAJ 2020; 192:E1802.
MCB Hughes et al. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med 2013; 158:781.
AR Young et al. Sub-optimal application of a high SPF sunscreen prevents epidermal DNA damage in vivo. Acta Derm Venereol 2018; 98:880.
CG Watts et al. Sunscreen use and melanoma risk among young Australian adults. JAMA Dermatol 2018; 154:1001.
AC Green et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257.
AR Heurung et al. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis 2014; 25:289.
J Wang et al. Recent advances on endocrine disrupting effects of UV filters. Int J Environ Res Public Health 2016; 13:782.
S Suh et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol 2020; 59:1033.
MK Matta et al. Effect of sunscreen application under maximal use conditions on plasma concentrations of sunscreen active ingredients: a randomized clinical trial. JAMA 2019; 321:2082.
MK Matta et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA 2020; 323:256.
JA Ruszkiewicz et al. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol Rep 2017; 4:245.
H Kang et al. Urinary metabolites of dibutyl phthalate and benzophenone-3 are potential chemical risk factors of chronic kidney function markers among healthy women. Environ Int 2019; 124:354.
Australian Government Department of Health. Therapeutic Goods Administration. Literature review on the safety of titanium dioxide and zinc oxide nanoparticles in sunscreens. Scientific review report. January 11, 2017. Available at: www.tga.gov.au/node/4309. Accessed July 8, 2021.
YH Mohammed et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol 2019; 139:308.
Valisure. Valisure detects benzene in sunscreen. May 25, 2021. Available at: https://bit.ly/2UdOUNO. Accessed July 8, 2021.
CA Downs et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3) on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch Environ Contam Toxicol 2016; 70:265.
FDA. Environmental Impact Statement (EIS) for certain sunscreen drug products. May 12, 2021. Available at: https://bit.ly/3weZrFT. Accessed July 8, 2021.
SL Schneider and HW Lim. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J Am Acad Dermatol 2019; 80:266.
D Fivenson et al. Sunscreens: UV filters to protect us: part 2-increasing awareness of UV filters and their potential toxicities to us and our environment. Int J Women’s Dermatol 2021; 7:45.
AS Paller et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92.
M Ghazipura et al. Exposure to benzophenone-3 and reproductive toxicity: a systematic review of human and animal studies. Reproductive Toxicol 2017; 73;175.
JC DiNardo and CA Downs. Can oxybenzone cause Hirschsprung’s disease? Reprod Toxicol 2019: 86:98.
RE Neale et al. The effect of sunscreen on vitamin D: a review. Br J Dermatol 2019; 181:907.
T Passeron et al. Sunscreen photoprotection and vitamin D status. Br J Dermatol 2019; 181:916.
AR Young et al. Optimal sunscreen use, during a sun holiday with a very high ultraviolet index, allows vitamin D synthesis without sunburn. Br J Dermatol 2019; 181:1052.
P Isedeh et al. Teaspoon rule revisited: proper amount of sunscreen application. Photodermatol Photoimmunol Photomed 2013; 29:55.
© The Medical Letter, Inc. All Rights Reserved.
This article has been freely provided.




