It is gratifying to see numbers continue to fall, but Covid 19 is still out there and I still hear reports of patients receiving prescriptions for Ivermectin. here is the latest from the Journal of the American Medical Association.
Here is the abstract or summary from the journal:
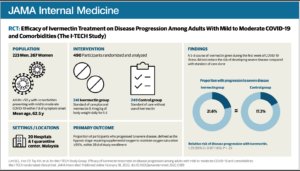
Efficacy of Ivermectin Treatment on Disease Progression
Among Adults With Mild to Moderate COVID-19
and Comorbidities
The I-TECH Randomized Clinical Trial
Steven Chee Loon Lim, MRCP; Chee Peng Hor, MSc; Kim Heng Tay, MRCP; Anilawati Mat Jelani, MMed;
Wen Hao Tan, MMed; Hong Bee Ker, MRCP; Ting Soo Chow, MRCP; Masliza Zaid, MMed; Wee Kooi Cheah, MRCP;
Han Hua Lim, MRCP; Khairil Erwan Khalid, MRCP; Joo Thye Cheng, MRCP; Hazfadzila Mohd Unit, MRCP;
Noralfazita An, MMed; Azraai Bahari Nasruddin, MRCP; Lee Lee Low, MRCP; Song Weng Ryan Khoo, MRCP;
Jia Hui Loh, MRCP; Nor Zaila Zaidan, MMed; Suhaila Ab Wahab, MMed; Li Herng Song, MD;
Hui Moon Koh, MClinPharm; Teck Long King, BPharm; Nai Ming Lai, MRCPCH;
Suresh Kumar Chidambaram, MRCP; Kalaiarasu M. Peariasamy, MSc; for the I-TECH Study Group
IMPORTANCE Ivermectin, an inexpensive and widely available antiparasitic drug, is
prescribed to treat COVID-19. Evidence-based data to recommend either for or against
the use of ivermectin are needed.
OBJECTIVE To determine the efficacy of ivermectin in preventing progression to severe
disease among high-risk patients with COVID-19.
DESIGN, SETTING, AND PARTICIPANTS The Ivermectin Treatment Efficacy in COVID-19
High-Risk Patients (I-TECH) study was an open-label randomized clinical trial conducted
at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and
October 25, 2021. Within the first week of patients’ symptom onset, the study enrolled
patients 50 years and older with laboratory-confirmed COVID-19, comorbidities, and mild
to moderate disease.
INTERVENTIONS Patients were randomized in a 1:1 ratio to receive either oral ivermectin,
0.4 mg/kg body weight daily for 5 days, plus standard of care (n = 241) or standard of care
alone (n = 249). The standard of care consisted of symptomatic therapy and monitoring
for signs of early deterioration based on clinical findings, laboratory test results, and
chest imaging.
MAIN OUTCOMES AND MEASURES The primary outcome was the proportion of patients who
progressed to severe disease, defined as the hypoxic stage requiring supplemental oxygen
to maintain pulse oximetry oxygen saturation of 95% or higher. Secondary outcomes of
the trial included the rates of mechanical ventilation, intensive care unit admission, 28-day
in-hospital mortality, and adverse events.
RESULTS Among 490 patients included in the primary analysis (mean [SD] age, 62.5 [8.7]
years; 267 women [54.5%]), 52 of 241 patients (21.6%) in the ivermectin group and 43 of
249 patients (17.3%) in the control group progressed to severe disease (relative risk [RR],
1.25; 95% CI, 0.87-1.80; P = .25). For all prespecified secondary outcomes, there were
no significant differences between groups. Mechanical ventilation occurred in 4 (1.7%) vs
10 (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17), intensive care unit admission in 6 (2.4%)
vs 8 (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79), and 28-day in-hospital death in 3 (1.2%)
vs 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09). The most common adverse event
reported was diarrhea (14 [5.8%] in the ivermectin group and 4 [1.6%] in the control group).
CONCLUSIONS AND RELEVANCE In this randomized clinical trial of high-risk patients with mild
to moderate COVID-19, ivermectin treatment during early illness did not prevent progression
to severe disease. The study findings do not support the use of ivermectin for patients with
COVID-19.
TRIAL REGISTRATION ClinicalTrials.gov Identifier: NCT04920942
JAMA Intern Med. doi:10.1001/jamainternmed.2022.0189
Published online February 18, 2022.