The big news of the day is the change in masking guidelines for vaccinated people to resume wearing masks indoors in areas where the virus is spreading widely. Unvaccinated people were supposed to wear them, but few did. No big surprise here. The recommendations two months ago were made based on data on the alpha variant. Now the more infectious delta variant has replaced it and is responsible for 80 +% of cases. If cases are rising, but vaccine rates are not, there are few other options. We can thank all those people out there who declined to be vaccinated. Overall, Texas is displayed as a state where transmission is widespread, but locally the risk is considered moderate. That may change tomorrow when local data is released at 4PM.
Here is the other Covid 19 news of the day as reported by HCA’s ( Hospital Corporation of America) infectious disease consultant Dr. Ed Septimus. Much of this contains technical terms but i will put my comments in there IN CAPS.
COVID-19 Updates
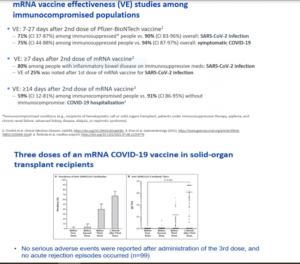
IMMUNOCOMPROMISED INDIVIDUALS
Several members of the ACIP https://www.cdc.gov/vaccines/acip/index.htmlsaid immunocompromised people would benefit from a COVID-19 booster shot during the
CDC panel’s July 22 presentation.
ACIP does not have the regulatory authority to officially recommend a booster shot, but it presented data showing that
a booster shot would help immunocompromised people prevent COVID-19.
Almost 3 percent of all U.S. adults are immunocompromised, including transplant recipients, some cancer survivors and
people with HIV, according to the CDC. Vaccines often are not as effective in immunocompromised people, as they
require their immune system to be stimulated in order to protect them against disease.
The presenters discussed four small studies involving transplant and dialysis patients who did not develop antibodies
after receiving their first two COVID-19 vaccine doses. After a third dose, 33 percent to 50 percent of them developed
antibodies to fight COVID-19. See slides from ACIP below and new article under Journal Review
Dr Septimus Comments: Almost every state is seeing an increase in COVID-19 hospitalizations, especially Nevada, Arkansas, Florida,
Texas, and Missouri. The only states not seeing hospitalizations grow are Maryland, North Dakota, Pennsylvania, Rhode
Island, and Vermont. The majority are in ages 18-55 (see above). 97% of admissions are in unvaccinated individuals.
The good news vaccinations rates are up 14% in the last week.
Interim Public Health Recommendations for Fully Vaccinated People July 21, 2021
Update: People who are immunocompromised should be counseled about the potential for reduced immune responses
to COVID-19 vaccines and to follow current prevention measures (including wearing a mask, staying 6 feet apart from
others they don’t live with, and avoiding crowds and poorly ventilated indoor spaces) to protect themselves against
COVID-19 until advised otherwise by their healthcare provider.
Comment: This is welcomed addition. In my previous commentary last Friday:
If you are older especially with underlying medical conditions or immune compromised your response to the vaccine
may not be as robust as the response in a younger person.
CDC now suggests this group should wear a mask and social distance when around others who you do not live with and
avoid crowds.
Department of Veterans Affairs, California, and NYC mandate Covid-19 vaccine for health care professionals
The Veteran Affairs becomes first federal agency to mandate vaccination, California the first state, and NYC the first big
city. I continue to be amazed at the low rate of vaccinations among certain HCWs.
Journal Review
Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant
Recipients With Minimal Serologic Response to 2 Doses JAMA published online July 23, 2021
doi:10.1001/jama.2021.12339
All kidney transplant recipients followed up in the outpatient between January 20,2021, and June 3,2021, with a
negative history for COVID-19 and SARS-CoV-2 antispike IgG levels less than 50 arbitrary units (AU)/mL on the day of the
first vaccine injection and 1 month after the second dose were included. All patients received a third vaccine dose
between April 9, 2021, and May 12, 2021.
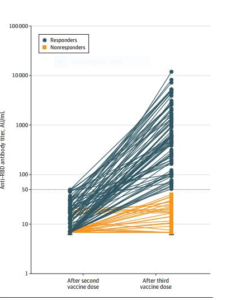
One month after the second dose, 159 kidney transplant recipients had IgG levels less than 50 AU/mL. The median age
was 57.6 years, 61.6% were men, and the median time from transplantation was 5.3 years. Ninety-five patients (59.7%)
had no antibody response after 2 doses (titers<6.8 AU/mL), and 64 patients (40.3%) showed a response below the
positivity limit (titers, 6.8-49.9 AU/mL). The third dose was injected a median of 51 days after the second dose. The
antibody response was measured a median of 28 days after the third vaccine injection, and 78 patients (49%) had
antibody levels greater than 50 AU/mL (median antibody titers of responders, 586 AU/mL; IQR, 197.2-1920.1 AU/mL).
Patients who had a weak response after the second dose were more likely to develop an antibody response after the
third dose compared with those without an antibody response (81.3% vs 27.4%, respectively; P = .001). Patients taking
tacrolimus, mycophenolate, and steroids were less likely to develop anti–SARS-CoV-2 antibodies than those treated with other regimens (35% vs 63%, respectively).
Comment: This study found that a third dose of Moderna vaccine induced a serologic response in 49% of kidney
transplant recipients who did not respond after 2 doses. The findings in this large group of kidney transplant recipients
are consistent with other studies of solid organ transplant
recipients. However, 51% of the patients did not develop anti– SARS-CoV-2 antibodies after the third dose, especially
those receiving triple immunosuppression. The major limitations of this study include that detailed B- and T-cell studies
were not performed, and the antibody level that correlates with protection is unknown. The results suggest a third dose
should be considered in organ transplant patients.
KEEP TAKING YOUR STATINS AND BLOOD PRESSURE MEDICATION. THEY MAY IMPROVE CHANCE OF SURVIVAL IF YOU GET COVID
Relation of prior statin and anti-hypertensive use to severity of disease among patients
hospitalized with COVID-19: Findings from the American Heart Association’s COVID-19
Cardiovascular Disease Registry PLOS ONE published online July 15, 2021
doi.org/10.1371/journal.pone.0254635
The investigators used data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 US
hospitals enrolled in the AHA’s COVID-19 cardiovascular disease (CVD) Registry to evaluate the associations between
statin use and outcomes.
Prior to admission, 42% of subjects (n = 4,449) used statins (7% on statins alone, 35% on statins plus anti-hypertensives).
Death (or discharge to hospice) occurred in 2,212 subjects (21%). Outpatient use of statins, either alone or with antihypertensives, was associated with a reduced risk of death (adjusted odds ratio [aOR] 0.59, 95% CI 0.50–0.69), adjusting
for demographic characteristics, insurance status, hospital site, and concurrent medications by logistic regression. In
propensity-matched analyses, use of statins and/or anti-hypertensives was associated with a reduced risk of death
among those with a history of CVD and/or hypertension (aOR 0.68, 95% CI 0.58–0.81). An observed 16% reduction in
odds of death among those without CVD and/or hypertension was not statistically significant. Comorbid conditions
were generally associated with increased risk of death in adjusted analyses. See below
Comment: Patients taking statins prior to hospitalization for COVID-19 had substantially lower odds of
death, primarily among individuals with a history of CVD and/or hypertension. These results are consistent with most
prior studies. As an observational study, this analysis is unable to prove causality. The investigators attempted to
account for confounders with both multivariable models and with propensity-score matched analyses, but the possibility
of residual confounding remains.
PEOPLE WITH REACTIONS TO THE FIRST VACCINE DOSE MAY TOLERATE A SECOND DOSE EVEN IF THEY HAD ANAPHYLAXIS
Safety Evaluation of the Second Dose of Messenger RNA COVID-19 Vaccines in Patients With Immediate
Reactions to the First Dose JAMA Intern Med published online July 26, 2021
doi:10.1001/jamainternmed.2021.3779
There is uncertainty as to whether to administer a second dose of mRNA COVID-19 vaccine after a first dose reaction. In
this study, the investigators examined the safety of the second dose of Pfizer or Moderna vaccine in those with a history
of immediate and potentially allergic reactions to the first dose.
This is a multicenter, retrospective study conducted by Massachusetts General Hospital ,Brigham and Women’s
Hospital ,Vanderbilt University Medical Center ,Yale School of Medicine, and University of Texas Southwestern Medical
Center from January 1, 2021, to March 31, 2021, included patients with an immediate allergic reaction to the Pfizer or
Moderna vaccine, which was defined as: (1) symptom onset within 4 hours of dose 1, (2) at least 1 allergic symptom, and
(3) referral for an allergy/immunology consultation with in-clinic or telehealth assessment . Anaphylaxis was scored
using the Brighton and the National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network
criteria. The primary outcome was second dose tolerance, which was defined as either: (1) no immediate symptoms
after second dose administration or (2) symptoms that were mild, self-limited, and/or resolved with antihistamines
alone.
COVID-19 vaccine first-dose reactions were evaluated, 130 (69%) were to Moderna and 59 (31%) to Pfizer. The most
frequently reported first-dose reactions were flushing or erythema (53 [28%]), dizziness or lightheadedness (49 [26%]),
tingling (46 [24%]), throat tightness (41 [22%]), hives (39 [21%]), and wheezing or shortness of breath (39 [21%]). Thirtytwo (17%) met anaphylaxis criteria. A total of 159 patients (84%) received a second dose. Antihistamine premedication
before the second dose was given in 47 patients (30%). All 159 patients, including 19 individuals with first-dose
anaphylaxis, tolerated the second dose. Thirty-two (20%) reported immediate and potentially allergic symptoms that
were associated with the second dose that were self-limited, mild, and/or resolved with antihistamines alone.
Comment: Although mild symptoms were reported in 20% of patients with second dose administration, all patients who
received a second dose safely completed their vaccination series and could potentially use mRNA COVID-19 vaccines in
the future if indicated. Second dose tolerance following reactions to the first dose argues that either many of these
initial reactions are not all truly allergic reactions, or supports an allergic, but non–immunoglobulin E–mediated
mechanism in which symptoms can typically be abated with premedications. When J&J vaccine received emergency use
authorization, the CDC recommended that individuals with an immediate and potentially allergic reaction to the first
dose of the Pfizer or Moderna mRNA COVID-19 vaccine could receive a J&J single as their second dose. With this
publication it may not be necessary to use J&J vaccine, but instead receive the second dose with a mRNA vaccine.
Ed Septimus, MD, FACP, FIDSA, FSHEA
Therapeutics Research and Infectious Disease Epidemiology, Department of Population Medicine
Harvard Medical School & Harvard Pilgrim Health Care Institute
eseptimus@gmail.com
Texas A&M College of Medicine
Houston, Texas




